A Highly Recyclable Self-Assembled Solid-State Electrolyte: Balancing Kevlar-Like Stability with Battery Performance
- Technical Research

- Sep 1, 2025
- 4 min read
Introduction
With the rapid growth of electric vehicle production, shortages of critical materials, and environmental concerns from end-of-life battery disposal, the importance of closed-loop battery recycling is increasingly evident. While significant progress has been made in battery recycling, including direct regeneration, direct recovery, and data-driven methods, numerous challenges persist: suboptimal cost-effectiveness, performance degradation after material recovery, and a lack of standardized solutions. Traditionally, recyclable materials often achieve this by incorporating reversible chemical bonds (such as ester, imine, or disulfide bonds). However, these bonds tend to be the weakest covalent links within the material, prone to failure under external stimuli, which can lead to reduced mechanical strength and compromised electrochemical performance.
To address this challenge, this study draws inspiration from the self-assembly principles of biological macromolecules, proposing intrinsically recyclable battery materials achieved through the self-assembly of small molecules and oligomers. This forward-thinking molecular design strategy aims to integrate recyclable chemistry at every level of the material—from individual battery cells to entire modules and packs—to balance both recycling efficiency and battery performance.
The Core Challenge: Performance Compromises in Traditional Recyclable Designs
In conventional material design, achieving recyclability often relies on introducing "dynamic covalent bonds" (e.g., ester, imine) into polymers that can break and reform under specific conditions (e.g., heat, light). However, these chemical bonds are frequently the least stable parts of the material structure. This can lead to issues such as insufficient mechanical strength and interfacial instability under actual operating conditions, ultimately affecting electrochemical performance and cycle life. The central challenge in this field has long been how to achieve material recycling without sacrificing performance.
A New Design Paradigm: Reversible Materials from Bio-Inspired Self-Assembly
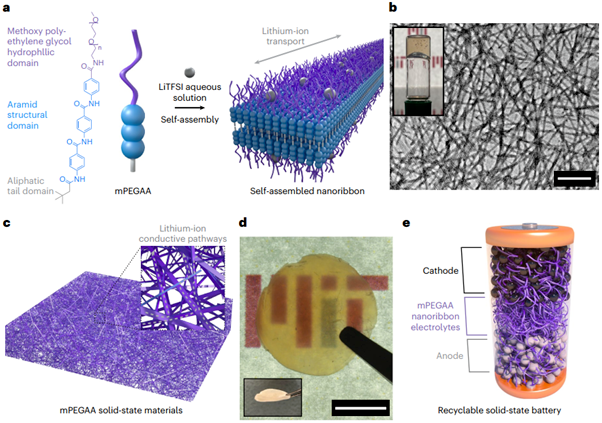
This research proposes a novel design paradigm: utilizing non-covalent interactions (e.g., hydrogen bonding, π–π stacking) to construct robust yet reversible materials. The research team designed an aramid amphiphile molecule called mPEGAA, based on the following core design principles:
Stable Core (Aramid Core): One part of the molecule mimics the structure of Kevlar fibers, comprising aromatic amide groups. These groups can form strong hydrogen bond networks and π–π stacking interactions, self-assembling into chemically inert and mechanically robust nanoribbon structures.
Conductive Shell (mPEG Shell): The other part of the molecule is methoxy poly(ethylene glycol) (mPEG). These flexible polymer segments are located on the surface of the nanoribbons and are responsible for conducting lithium ions.
This design cleverly decouples the functions of "structural stability" and "ionic conductivity" at the molecular level, forming a unique material that combines the advantages of both.
Key Performance Metrics: Mechanical Strength and Ionic Conductivity
By hot-pressing the self-assembled mPEGAA nanoribbons, the research team fabricated flexible, self-supporting solid-state electrolyte membranes. Performance tests showed that this material exhibited outstanding overall properties:
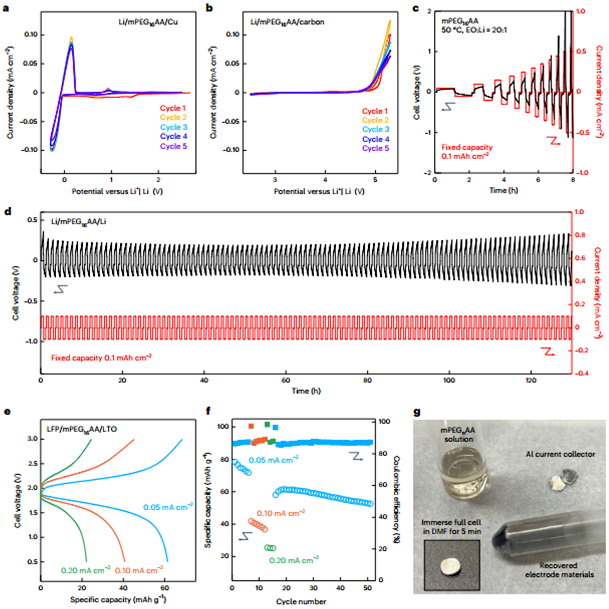
Exceptional Mechanical Properties: Benefiting from its Kevlar-like internal structure, the solid-state electrolyte demonstrated a strength of up to 70 MPa and a toughness of 0.95 MJ·m⁻³, sufficient to withstand stresses during battery manufacturing and application.

High Ionic Conductivity: The mPEG chain segments on the nanoribbon surface provide an efficient pathway for lithium ion transport. At 50°C, the material achieved an ionic conductivity of 1.6 × 10⁻⁴ S·cm⁻¹ with a lithium ion transference number of 0.42.
Wide Electrochemical Window: The electrolyte exhibits a wide electrochemical stability window of approximately 4.5 V and shows good compatibility with lithium metal anodes, supporting stable lithium deposition/stripping.

Recyclability Validation: Efficient and Complete Component Separation
The most prominent advantage of this material lies in its unique "disposal-on-demand" recycling mechanism. Since the entire nanoribbon structure is maintained through non-covalent interactions, at the end of the battery's life, these interactions can be disrupted by simply soaking the battery in common organic solvents (such as DMF or methanol).
This process allows the robust solid-state electrolyte membrane to rapidly and completely dissolve, enabling the intact separation of core battery components like cathode and anode materials and conductive agents. This facilitates efficient and low-cost closed-loop recycling.
Conclusion and Outlook: Paving a New Path for Sustainable Batteries
This study successfully developed a recyclable solid-state electrolyte based on molecular self-assembly, with a design philosophy that overcomes the traditional conflict between performance and recyclability. By mimicking the wisdom of biological macromolecules, this work demonstrates that sophisticated molecular design can create advanced battery materials that are both robust, high-performing, and easily recyclable at the end of their life.
This achievement not only provides a concrete and feasible technical solution for current battery recycling challenges but also opens a new and highly promising avenue for the future development of green, sustainable energy storage materials.
Literature Information
Cho, Y., Fincher, C.D., Lamour, G. et al. Reversible self-assembly of small molecules for recyclable solid-state battery electrolytes. Nat. Chem. (2025). https://doi.org/10.1038/s41557-025-01917-6
Author Biographies
First and Corresponding Author: Yukio Cho Stanford Energy Postdoctoral Fellow (2024), Ph.D. in Materials Science from MIT (2023). His research focuses on nanomaterials and electrochemical applications.
Corresponding Author: Professor Yet-Ming Chiang Professor in the Department of Materials Science and Engineering at MIT. His research aims to design, synthesize, and characterize advanced materials and electrochemical devices for clean energy technologies, including low-carbon transportation, grid-scale energy storage, and sustainable manufacturing.
Corresponding Author: Associate Professor Julia H. Ortony Associate Professor in the Department of Chemistry and Biochemistry at the University of California San Diego. Her research focuse




Comments