Beyond Lithium Metal: Li-Ga Compound Anodes Enable Dendrite-Free All-Solid-State Batteries
- Technical Research

- Dec 22, 2025
- 3 min read
Introduction
While All-Solid-State Batteries (ASSBs) promise a revolution in energy storage, the "Holy Grail" anode—Lithium Metal—remains plagued by dendrite growth and interfacial instability, even within solid electrolytes. Alternative strategies like carbon or alloy anodes often compromise on coulombic efficiency or suffer from volume expansion.
Recently, a research team led by Prof. Cheol-Min Park at Kumoh National Institute of Technology published a breakthrough in Advanced Energy Materials. They utilized Density Functional Theory (DFT) to screen and identify a specific Lithium-Gallium phase (LiGa) that acts as a highly conductive, thermodynamically stable anode. This material effectively suppresses dendrites while maintaining high-rate performance, offering a robust solution for high-performance ASSBs.
Screening for the Perfect Phase: Why LiGa?
The team began by systematically evaluating various Li-Ga compounds using DFT calculations and binary phase diagrams.
Thermodynamic Stability: The Convex Hull analysis identified Li₃Ga₁₄, LiGa, and Li₂Ga as stable phases. Among them, LiGa exhibited the lowest formation energy (-0.505 eV/atom).
Conductivity: Electronic structure analysis revealed that LiGa possesses metallic character, ensuring excellent electronic conductivity, while also offering rapid Li-ion diffusion channels.
Experimental Validation: The team successfully synthesized these phases via mechanochemistry. Electrochemical tests confirmed that LiGa demonstrated the best stability (critical current density of 3.3 mA cm⁻²) and the highest ionic conductivity among the candidates.

Superior Stability: Outperforming Li-Metal and Li-In
When pitted against traditional Lithium Metal and Lithium-Indium (Li-In) anodes, LiGa showed distinct advantages:
Vs. Li-Metal: Li-metal failed due to dendrite short-circuits.
Vs. Li-In: Li-In suffered from high voltage hysteresis and capacity decay at high rates.
LiGa Performance: The LiGa symmetric cells maintained stable cycling for over 1000 hours with the lowest and most stable interfacial impedance.

Full Cell Performance: High Loading & Fast Charging
The practical viability of LiGa was tested in full cells paired with an NCM622 cathode under an optimized stack pressure of 3 MPa.
High Loading: The cells remained highly reversible even at high cathode loadings (up to 50 mg cm⁻²).
Durability: At a loading of 30 mg cm⁻², the cell retained >80% capacity after 160 cycles. Even at 50 mg cm⁻², it retained 94% after 100 cycles.
Fast Charging: The system demonstrated excellent reversibility under fast-charge conditions ranging from 1C to 10C.

Mechanism and Interface: The Secret to Dendrite Suppression
Why is LiGa so stable?
Storage Mechanism: Unlike the plating/stripping of Li-metal, LiGa operates via an interstitial mechanism where excess Lithium occupies the lattice interstices to form Li₁₊ₓGa (x≤0.8). This process involves no phase transition, ensuring structural integrity.
Interface Evolution: Cross-sectional analysis showed that while Li-metal interfaces degraded severely with dendrites, the LiGa interface remained dense and pristine after 100 cycles. The LiGa anode forms a stable interface with the sulfide electrolyte (Li₆PS₅Cl), generating only trace amounts of Li₂S, which helps maintain low resistance.

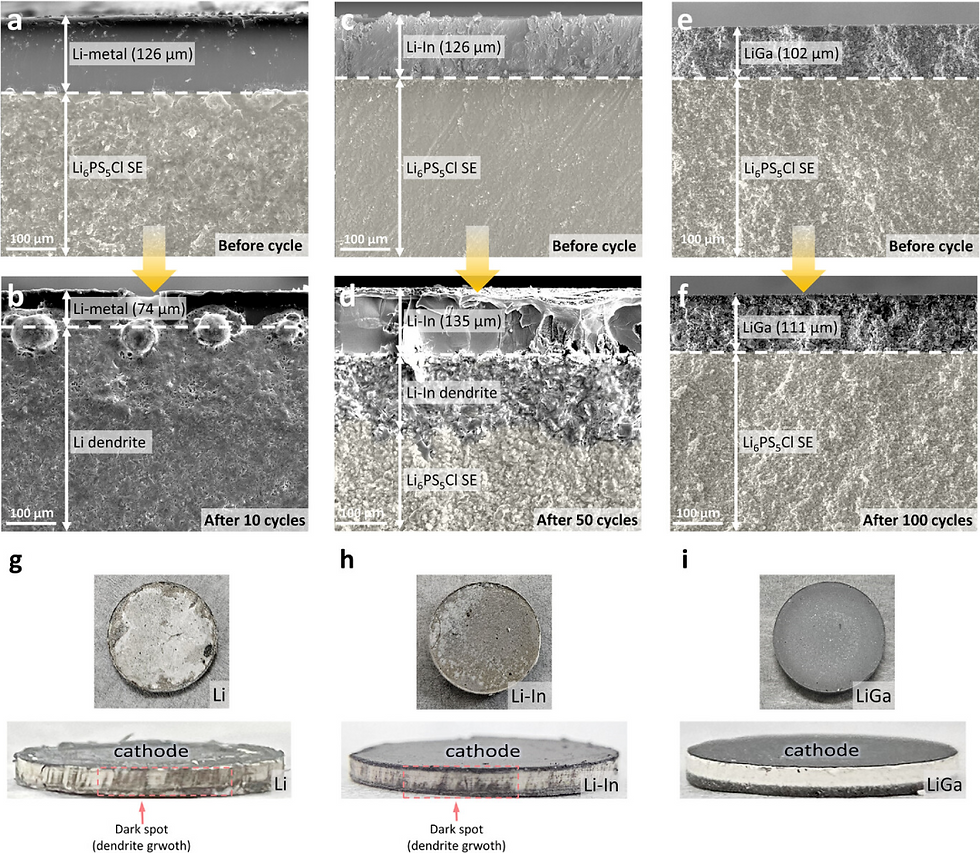

Conclusion
This study establishes LiGa as a superior anode candidate for all-solid-state batteries. By combining high ionic/electronic conductivity with a unique interstitial storage mechanism, LiGa solves the critical issues of dendrite growth and interfacial instability, paving the way for safer, high-energy-density batteries.
Literature Information
J.-M. Yoon, et al., "Highly Conductive and Dendrite-Free Li–Ga Compound Anodes for High-Performance Lithium All-Solid-State Batteries." Advanced Energy Materials (2025): e05248. https://doi.org/10.1002/aenm.202505248





Comments