A New Strategy for Fast-Charging Anodes: Why is Atomic "Disorder" More Critical Than Initial "Conductivity"?
- Technical Research

- Sep 8, 2025
- 4 min read
Introduction
Fast charging is one of the core technologies determining the user experience and market adoption of electric vehicles (EVs). However, conventional graphite anodes face safety issues like lithium dendrite formation under fast-charging conditions, limiting their application.
Consequently, developing new anode materials that combine high-rate performance with safety has become a major focus for the industry. Niobium oxides with Wadsley–Roth layered structures have shown promise, but their low intrinsic electronic conductivity has long been considered a performance bottleneck.
A recent study published in the Journal of the American Chemical Society challenges this conventional wisdom through an ingenious comparative experiment. The research demonstrates that, in certain advanced anode materials, suppressing lithium-ion "ordering" is more critical than enhancing initial electronic conductivity, proposing "atomic disorder" as a new design strategy to improve fast-charging kinetics.
An Ingenious Comparison: Ordered Metal vs. Disordered Insulator
To investigate the core factors influencing fast-charging performance, the research team selected two materials with highly similar structures but starkly different electronic properties for a head-to-head comparison:
Nb₁₂O₂₉: A structurally regular, atomically ordered material that is metallic in its pristine state.
Ti₂Nb₁₀O₂₉: A material with a disordered structure due to the intermingling of Ti/Nb atoms, making it an insulator in its pristine state.
Conventional wisdom would suggest that the metallic Nb₁₂O₂₉ should exhibit superior electrochemical performance. However, the experimental results overturned this prediction.
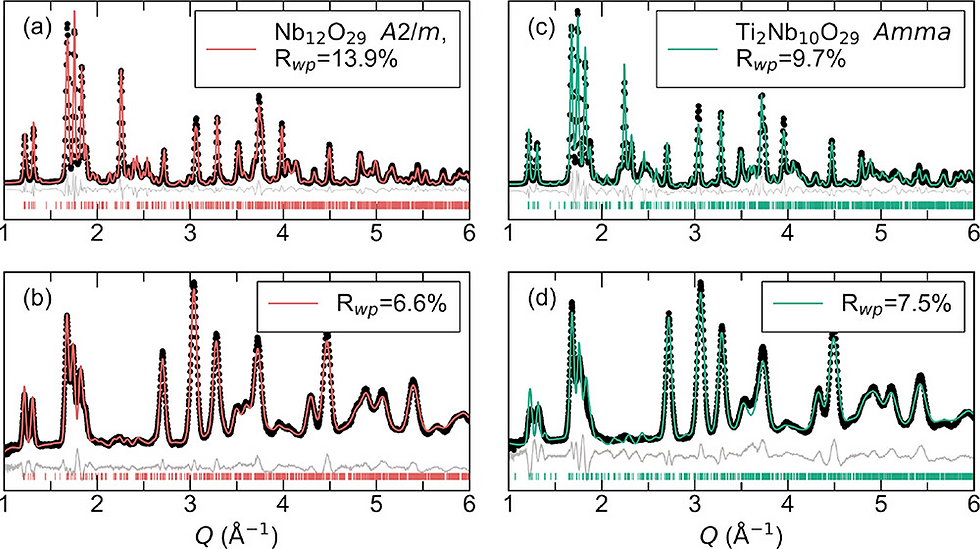

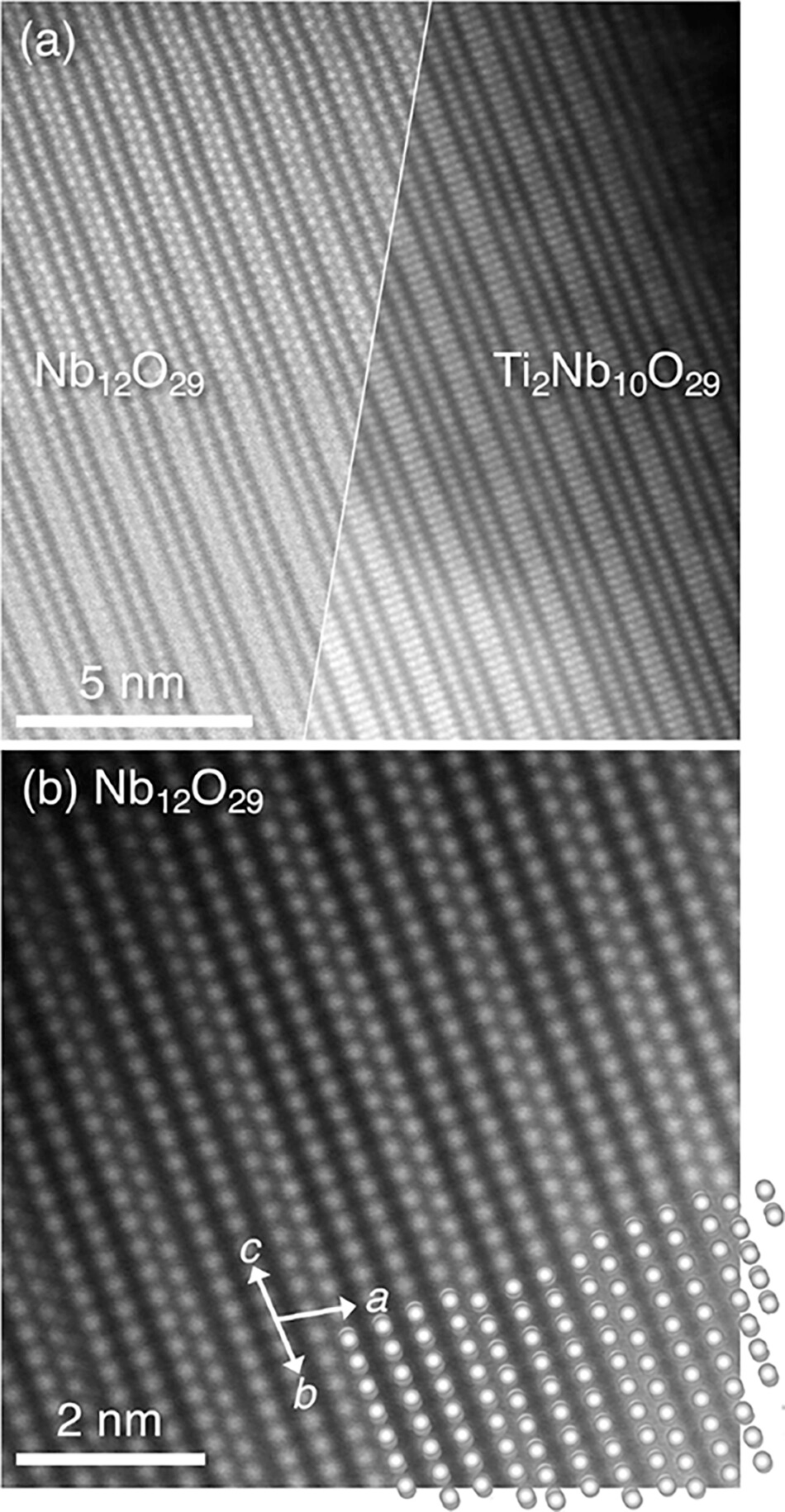

The Surprising Result: The Disordered Insulator Pulls Ahead at High Rates
At low rates (slow charge/discharge), both materials delivered similarly high capacities. However, as the charging rate increased, their performance began to diverge. The results showed that the initially insulating Ti₂Nb₁₀O₂₉ demonstrated significantly better capacity retention at 1C and higher rates than the metallic Nb₁₂O₂₉.
This counter-intuitive outcome indicates that initial electronic conductivity is not the sole, or even the primary, determining factor for the fast-charging performance of this class of materials.


Uncovering the Real Bottleneck: The Entropy Barrier of Li-ion "Ordering"
The study found that although Ti₂Nb₁₀O₂₉ starts as an insulator, it rapidly undergoes an "insulator-to-metal transition" during the first lithiation, gaining excellent electronic conductivity and thereby eliminating the initial conductivity gap with Nb₁₂O₂₉.
The true performance difference originates from the behavior of lithium ions during the intercalation process. Through entropy potential measurements and other advanced analyses, the research revealed:
In the atomically ordered Nb₁₂O₂₉, the intercalated lithium ions tend to arrange themselves into regular, ordered structures. Forming this ordered configuration requires overcoming an additional energy barrier (an "entropy barrier"), which acts as a "speed bump" under fast-charging conditions and limits the kinetics.
In the atomically disordered Ti₂Nb₁₀O₂₉, the random distribution of Ti/Nb atoms disrupts the tendency of lithium ions to form ordered arrangements. The lithium ions intercalate in a more flexible "solid-solution" manner, avoiding the energy barrier associated with ordering and thus exhibiting superior fast-charging kinetics.



The Performance Trade-off: Rate Capability vs. Long-Term Stability
While the disordered structure provides an advantage in rate performance, the study also identified a potential drawback. Long-term cycling tests showed that the ordered Nb₁₂O₂₉ exhibited better capacity retention. XPS analysis revealed that the disordered Ti₂Nb₁₀O₂₉ forms a higher proportion of unstable Nb³⁺ species upon deep lithiation, which may be a reason for its slightly inferior long-term stability.

Conclusion & Design Implications: Using "Disorder" as a Design Tool
The core conclusion of this research is that for certain high-rate electrode materials, introducing atomic-level "disorder" to suppress Li-ion ordering during charge/discharge is a more effective strategy for optimizing fast-charging performance than solely pursuing high initial electronic conductivity.
"Disorder" can break the entropy barrier created by lithium-ion ordering, thereby significantly improving kinetic performance. This finding provides a novel and universally applicable concept for the design of high-performance, fast-charging anode materials: actively using "atomic disorder" as a tunable design tool to achieve optimal electrochemical properties.
The exploration of next-generation fast-charging anodes extends beyond niobium oxides and is a core focus of the battery industry's technological evolution.
In this arena, LIMX Power has achieved a key breakthrough, having now mass-produced and commenced bulk deliveries of the industry's first all-silicon-carbon anode battery.
Literature Information
Metallicity, Atomic Disorder, and Li-Ion Storage in Fast-Charging Anodes, Kira E. Wyckoff, Arava Zohar, Tianyu Li, Yucheng Zhou, Linus Kautzsch, Welton Wang, Ananya Kepper, Ashlea R. Patterson, H. Cein Mandujano, Krishna Prasad Koirala, Anna Kallistova, Wenqian Xu, Jue Liu, Laurent Pilon, Anthony K. Cheetham, and Ram Seshadri, Journal of the American Chemical Society Article ASAP, DOI: 10.1021/jacs.5c06578.




Comments