Iron Battery Renaissance: Prof. Yi Cui's Team Cuts Costs by 90% with "Urea-Enabled" High-Concentration Electrolytes
- Technical Research

- Dec 11, 2025
- 3 min read
Introduction
In the blueprint for massive grid-scale energy storage, Iron stands out as one of the most attractive candidates. It ranks fourth in crustal abundance, costs only 4% as much as zinc, and boasts a high theoretical specific capacity of 960 mAh/g. However, the commercialization of iron metal batteries has long been stalled by a persistent issue: Hydrogen Evolution Reaction (HER). In traditional electrolytes, iron reacts with water to generate hydrogen gas, leading to low efficiency and dendrite growth.
While the "Water-in-Salt" (high concentration) strategy effectively inhibits water activity, cheap ferrous sulfate (FeSO₄) has low solubility (limited to 1.4 M), preventing high concentrations. Conversely, ferrous chloride (FeCl₂) is highly soluble but suffers from severe corrosion issues.
Recently, Prof. Yi Cui's team at Stanford University published a breakthrough in Nature Communications. They innovatively introduced Urea as a "hydrotrope" to break the solubility limit of ferrous sulfate, constructing a low-cost, environmentally friendly, and high-performance high-concentration iron-based electrolyte.
Core Breakthrough: Hydrotropes Shatter the Solubility Ceiling
The core of this research lies in the "hydrotropic effect" of urea. Traditional ferrous sulfate has a solubility limit of only 1.4 M in water, which is insufficient to form a high-concentration system that suppresses water activity.
The team discovered that urea molecules act as a "lubricant" to reconstruct the hydrogen bond network in the solution. By introducing urea, the solubility of ferrous sulfate was astonishingly boosted to 10 M (termed SHCE-10 m). This not only achieves high concentration but also completely avoids the use of corrosive chloride systems.

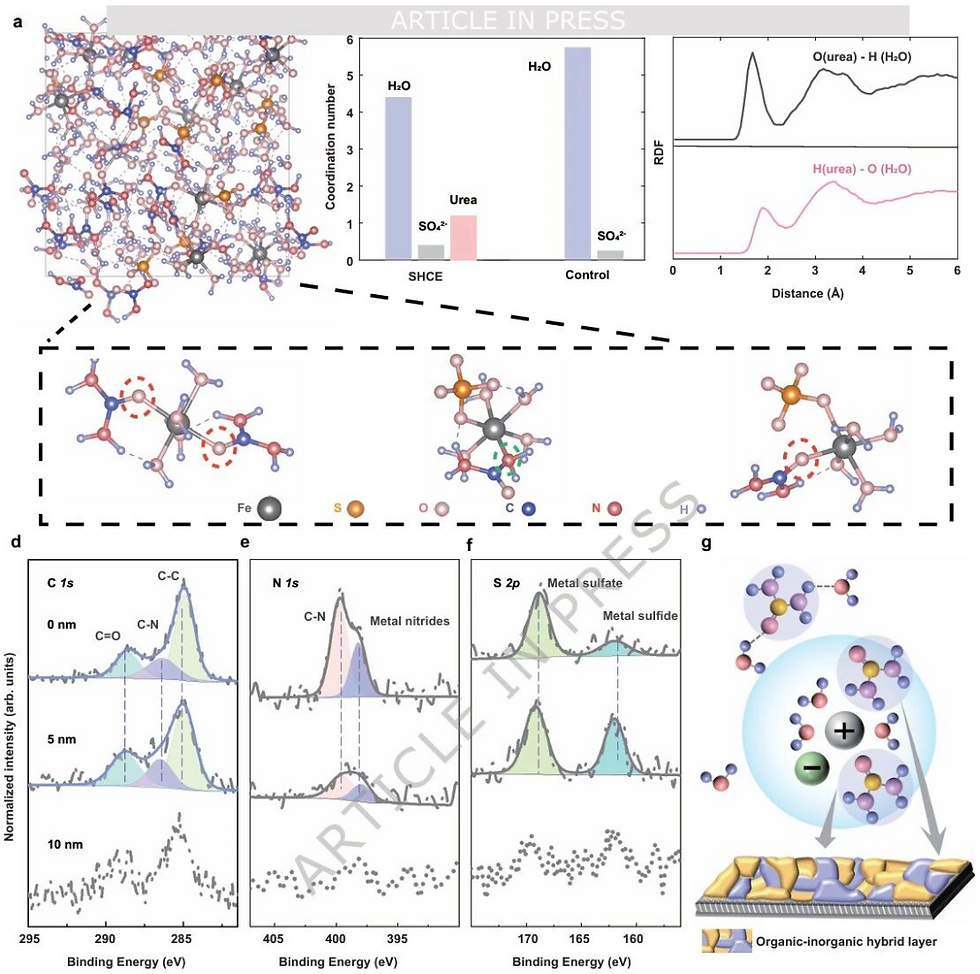
Unveiling the Mechanism: Reshaping the Microscopic "Social Circle"
Why does adding urea inhibit hydrogen evolution? Raman spectroscopy and molecular dynamics (MD) simulations revealed the microscopic mechanism:
Competing for Coordination: In the high-concentration system, urea molecules and sulfate ions (SO₄²⁻) force their way into the iron ion (Fe²⁺) solvation sheath, "crowding out" the original water molecules. The coordination number of water around Fe²⁺ drops from 5.8 to 4.4.
Locking Free Water: Urea forms a new, strong hydrogen bond network with water molecules, significantly reducing the activity of free water.
This reshaping of the micro-environment reduces the HER overpotential by 0.4 V, curbing hydrogen generation at the source.
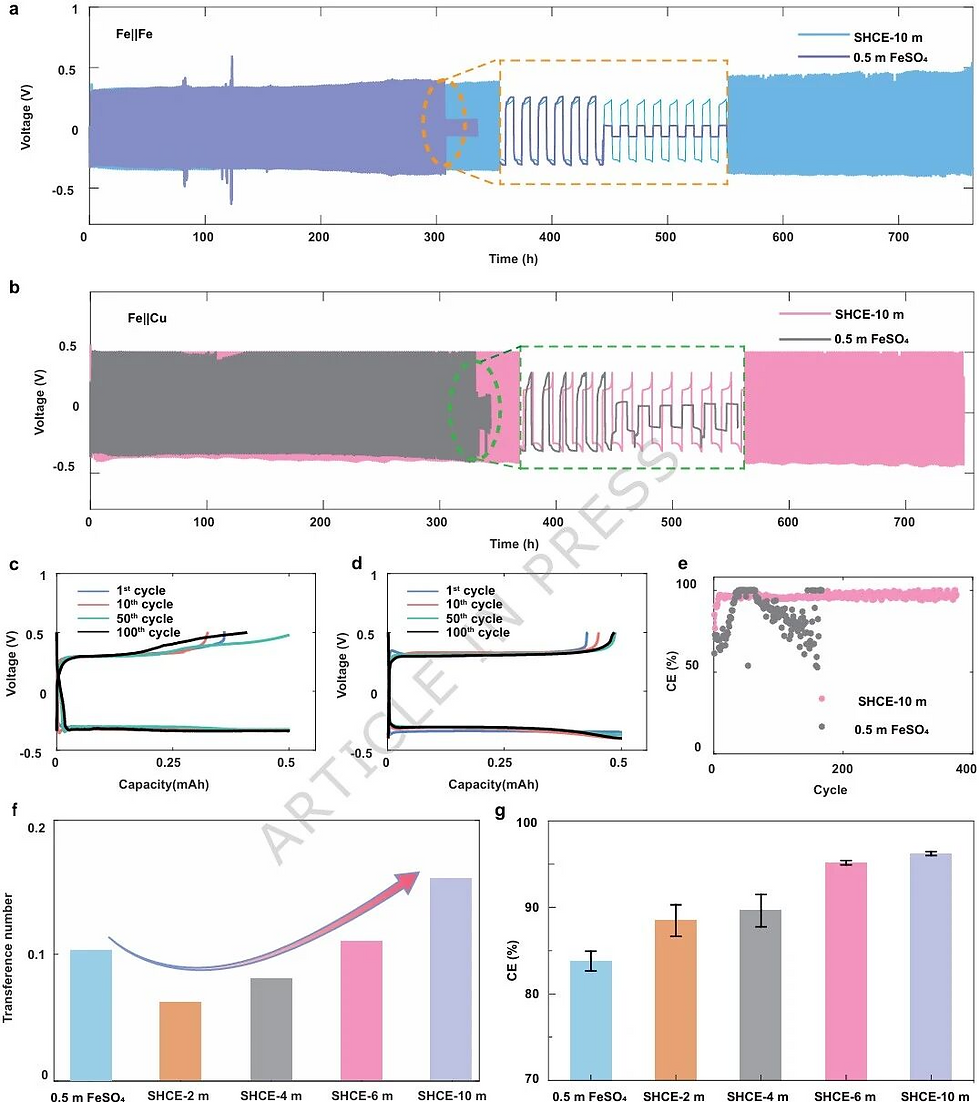
Performance Leap: Goodbye Bubbles and Dendrites
The improvement in macroscopic performance is visible and significant:
Suppressed Hydrogen Evolution: In-situ optical microscopy showed that traditional electrolytes generated massive bubbles within 2 minutes of applying current, whereas the new electrolyte showed almost no bubbles even after 8 minutes. Gas chromatography confirmed that cumulative hydrogen generation was only 5% of that in traditional electrolytes.
Smooth Deposition: Under SEM, iron deposition in traditional electrolytes appeared loose and porous (prone to oxidation and detachment). In contrast, deposition in the new electrolyte presented as dense, flat, cubic crystal stacks with no dendrite growth.

Economics & Stability: Scaling Up
Electrochemically, the SHCE-10 m electrolyte boosted the Coulombic Efficiency (CE) of the iron anode from 84.6% to 96.5% and achieved stable cycling for over 300 hours, whereas the control group failed due to short circuits in just 150 hours.
Even more exciting is the economic outlook. Compared to expensive salts currently under research (like triflates), this system uses extremely cheap urea and ferrous sulfate, reducing electrolyte raw material costs by approximately 90%. This provides a feasible, cost-effective technical path for the large-scale application of iron metal batteries in grid storage.
Literature Information
Hydrotrope-enabled high concentration aqueous electrolytes for reversible and sustainable iron metal anodes; Nature Communications; 2025; 10.1038/s41467-025-65160-w.




Comments